Case 20.3
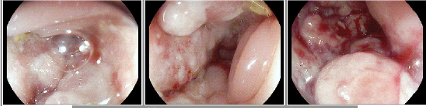
Vedolizumab was discontinued by gastroenterology. However, the rheumatologist was concerned about tofacitinib safety and stopped it. She was re-challenged with infliximab. After the first dose, she was found to have an undetectable serum drug concentration and detectable antibody to infliximab. She was admitted to the hospital with dehydration and fatigue. A sigmoidoscopy demonstrated the following:
Colonoscopy Findings: Severe stricturing disease at the ileocecal valve and spontaneous bleeding.
IV methylprednisolone was started. A combination of ustekinumab and vedolizumab was considered as was a trial of golimumab. As the first two were initially ineffective and the later class was associated with significant infection, it was not pursued. After extensive discussion with the patient and her family, with shared decision making, she was started on upadacitinib 45 mg daily, reduced to 30 mg per day and is currently in endoscopic and symptomatic remission.